Small RNA Sequencing
Purify small RNA
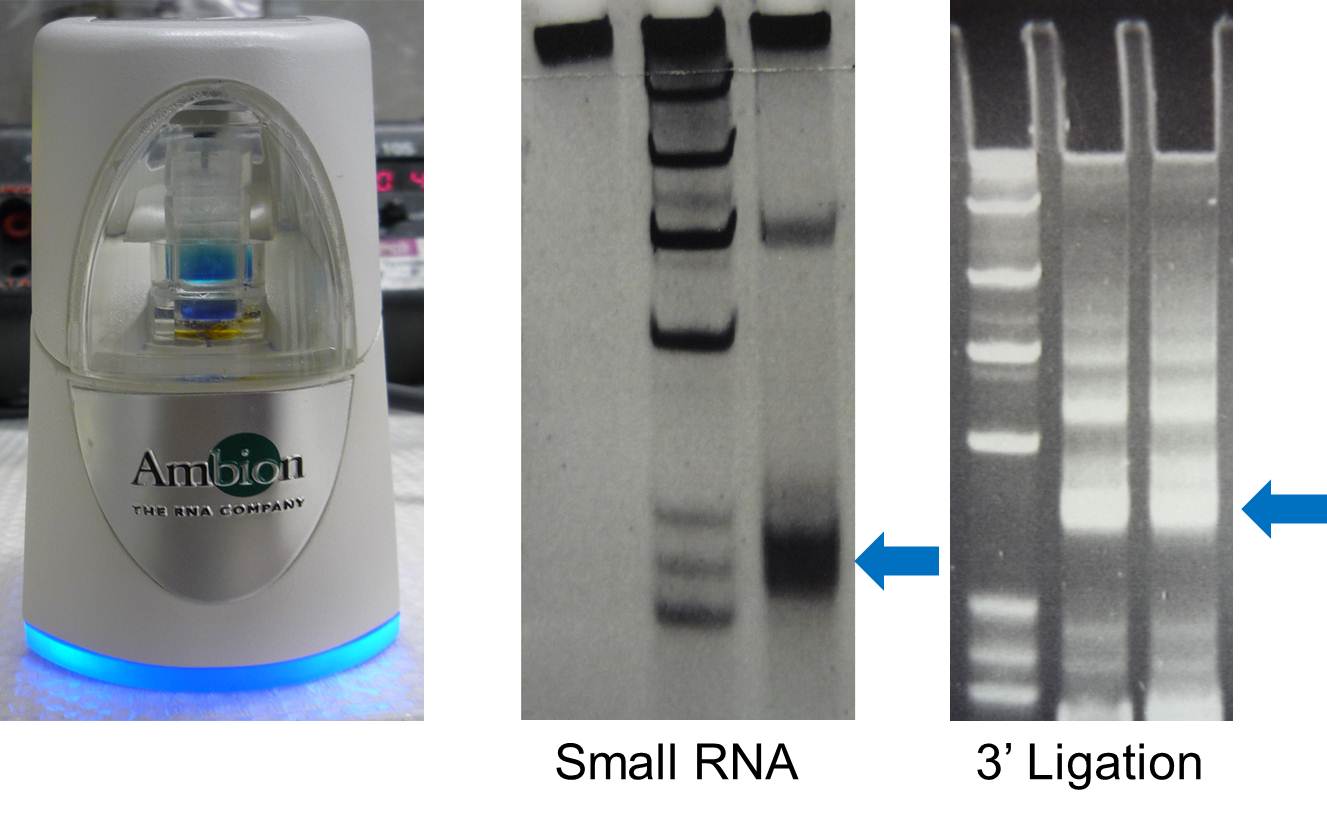
- 1. Use FlashPAGE (Ambion) to purify small RNA fraction, directly go to step 17.
- 2. Alternatively, cast a 1.5mm 15% UREA TBE PAGE, using 5 wells comb.
- 3. Pre-run the gel at 200V for 15 min.
- 4. Load 20-100 ug of total RNA with equal volume of formamide loading buffer (95% formamide, 2x TBE, 0.001% bromophenol blue, 0.001% Orange G).
- 5. Run the gel at 200V until the Orange G dye just runs out.
- 6. Stain the gel with SYBR gold for 10 min in the dark.
- 7. Cut the sRNA bands under blue light.
- 8. Crush the gel by spinning the gel slice through a punctured 0.5 ml epp tube placed in a 2 ml epp tube.
- 9. Resuspend the gel powder in 1 mL RNase-free TE with 0.3M NaAc pH 5.2, rotate overnight at 4C.
- 10. Spin down the gel debris and recover the solution containing sRNA.
- 11. Optional: extract with 2-butanol a few times to remove excessive water if necessary.
- 12. Add 2 ul glycogen (20mg/ml).
- 13. Extract with equal volume of chloroform, invert the tube for several times, spin at 14,000rpm, 5 min, 4C.
- 14. Move the aqueous layer to a new tube, add 3 volumes of 100% EtOH, freeze at -20 C for 0.5 h and spin down the sRNA (14,000rpm, 30mins, 4C).
- 15. Discard the supernatant, wash the pellet with 500 uL of 75% EtOH, spin the sample at 14,000rpm, 10 mins, 4C, repeat the washing one more time.
- 16. Air-dry the pellet, dissolve the pellet in 10 – 15 ul DEPC-H2O, roughly determine the concentration of the small RNA (Invitrogen picogreen assay kit).
3' linker ligation
- 17. Ligate preadenylated 3' linker to small RNA using Truncated T4 RNA ligase 2 (NEB).
- 18. Incubate the reaction mix at 22C for 5h or 18C overnight.
- 19. Mix the ligation product with 10 uL of formamide loading buffer.
- 20. Run Urea-PAGE gel at 200V until the Orange G dye runs out.
- 21. Stain the gel with SYBR gold in the dark for 10 min.
- 22. Cut and purify the ligation product (indicated by arrow).
- 23. Incubate the reaction mix at 22C for 5h or 18C overnight.
- 24. Mix the ligation product with 10 uL of formamide loading buffer.
- 25. Run Urea-PAGE gel at 200V until the Orange G dye runs out.
- 26. Stain the gel with SYBR gold in the dark for 10 min.
- Cut and purify the ligation product (indicated by arrow).
5' linker ligation
- 27. Use T4 RNA ligase 1 (Enzymatics) to ligate 5'SR adapter (RNA oligo) to the small RNA with 3' linker. The blocking group in the 3'SR can prevent small RNA self-ligation (circulation) during 5' ligation.
- 28. Incubate the reaction mix at 25C for 5h or 18C overnight.
Reverse Transcription
- 29. Add 0.5 uL (2 uM) RT primer to the ligation product, immediately transfer to 60C PCR block for 30 s, incubate on ice and prepare the RT reaction as follow:
| sRNA ligation product with RT primer | 20μL |
| dNTP (10 mM) | 1.5 uL |
| DTT (0.1 M) | 2 uL |
| SuperScriptIII buffer 5x | 6 uL |
| SuperScriptIII | 0.5 uL |
- 30. Perform RT reaction at 50C for 1 h, heat inactivate at 70C for 15 min.
PCR enrichment
- 31. Pool all indexed sRNA libraries for PCR:
| RT product (from step 28 or 29) | 10 uL |
| Primer (10 uM) | 1 uL |
| 5x Phusion HF Buffer | 8 uL |
| 10 mM dNTP | 1 uL |
| H2O | 19.5 uL |
| Phusion II | 0.5 uL |
- 32. Perform PCR as follow, if the library is over-amplified or not visible, used the remaining RT product and adjust PCR cycle accordingly.
- 33. Purify sRNA library from the gel, quantify and QC using Agilent bioanalyzer.
|